Proteins play a central role in virtually every biological process, making the study of their expression and function critical to advancing medicine and biology. However, human tissues are complex ecosystems composed of many different cell types intricately organized within microscopic structures. When analyzing protein expression from bulk tissue samples, this complexity can mask important cell-specific protein signals, limiting our understanding of tissue biology. Laser microdissection (LMD) is a transformative technology that addresses this challenge by allowing researchers to selectively isolate microscopic regions or specific cells from tissue sections with unparalleled precision.
Understanding Laser Microdissection
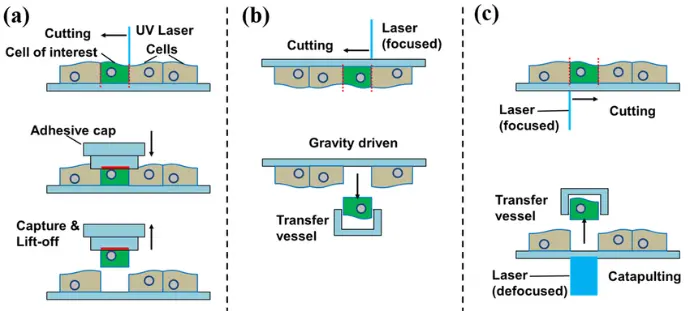
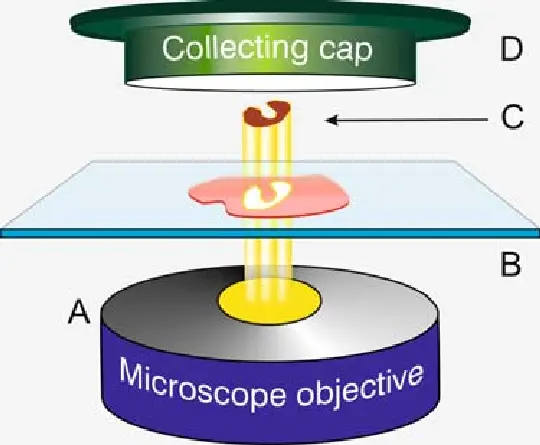
Laser microdissection refers to a group of microscopic techniques that use focused laser beams to cut or capture defined areas from thin tissue sections mounted on slides. The process is performed under high-resolution microscopy, enabling the operator to visually identify regions of interest based on morphology or staining characteristics before precise dissection.
The two main approaches include:
Laser Capture Microdissection (LCM): This method uses an infrared or ultraviolet laser to activate a thermoplastic film placed over the tissue section. The film selectively adheres to the targeted cells, which are then lifted off the slide.
- Laser Cutting Microdissection: A focused laser beam cuts around the perimeter of the desired tissue region, which falls into a collection vessel positioned below the slide or is propelled into a tube by laser pressure catapulting.
Why is Laser Microdissection Essential for Proteomics?
Proteomics, the large-scale study of proteins, demands samples that are as pure and representative as possible to detect and quantify proteins reliably. In heterogeneous tissues, contaminating cells can dilute rare protein signals, obscure differential expression, or introduce noise.
Laser microdissection provides critical advantages for proteomic research:
Cellular Precision: Isolates pure populations of cells or micro-anatomical regions based on morphology or biomarker staining, ensuring sample homogeneity.
Retention of Molecular Integrity: Especially when combined with optimized tissue preservation methods, LMD samples maintain high-quality proteins suitable for mass spectrometry and other sensitive techniques.
Use of Archival Samples: LMD works effectively on formalin-fixed paraffin-embedded (FFPE) tissues, which constitute vast, valuable archives of clinical samples with associated patient data.
Linking Molecular and Histological Data: The ability to target specific histological features allows proteomic data to be directly correlated with tissue architecture and pathology.
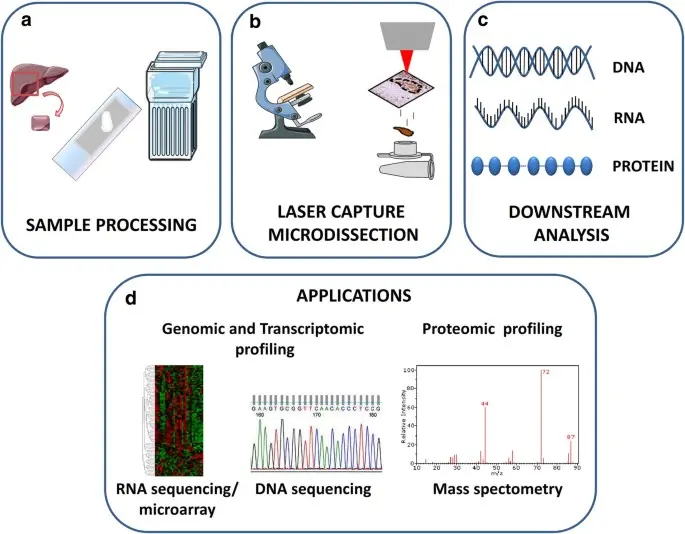
The Laser Microdissection Workflow: Step-by-Step
Sample Preparation:
Tissue blocks are thinly sliced (5-10 microns thick) and mounted onto specialized membrane coated slides compatible with LMD instruments. Sections are stained lightly to reveal cellular morphology or immunostained to highlight markers of interest.
Microscopic Visualization:
Under an advanced microscope integrated with laser microdissection hardware, the operator navigates the slide to locate areas or cells to isolate. The use of digital imaging and annotation tools allows precise targeting.
Laser Dissection or Capture:
Depending on the system, the laser either cuts the perimeter of the target region or activates a film to capture the cells. The non contact nature of the laser preserves sample integrity and prevents contamination.
Sample Collection:
The microdissected tissue is collected into tubes or caps for protein extraction. This material, though minute in quantity, is enriched for the specific cells or structures of interest.
Downstream Proteomic Analysis:
Proteins are extracted and digested into peptides, which are then analyzed by mass spectrometry or other proteomic platforms. Data can be integrated with clinical metadata and histological images.
Advantages of Laser Microdissection
Laser microdissection (LMD) offers several distinct advantages that make it an indispensable tool in modern molecular biology and tissue proteomics:
High Precision:
LMD enables the targeted selection and isolation of microscopic regions, down to individual cells or even subcellular structures. This precision is especially beneficial when analyzing heterogeneous tissue samples, where only specific regions are relevant for molecular analysis. The laser-guided extraction ensures that researchers can focus exclusively on areas of interest without compromising neighboring tissue.
No Contamination:
Unlike manual dissection methods, LMD operates without physical contact. The non-contact, laser-based system minimizes the risk of sample contamination from blades, tools, or external handlers. This purity is crucial when studying low-abundance molecules, as even slight contamination can alter results.
Compatibility with Archival Samples:
LMD works effectively on both frozen and formalin-fixed paraffin-embedded (FFPE) tissue sections. Since FFPE blocks are widely available in biorepositories, this compatibility greatly expands the range of accessible samples for retrospective and longitudinal studies.
Preserved Molecular Integrity:
Carefully microdissected regions retain critical molecular components such as proteins, DNA, and RNA suitable for downstream applications. Laser cutting preserves sample morphology and does not thermally damage the biomolecules within the tissue, allowing for high-quality extraction and analysis.
Applications in Tissue Proteomics
In tissue proteomics, sample purity is key. Laser microdissection provides researchers with a method to collect homogenous populations of cells directly from complex tissue environments. By isolating well-defined regions, LMD enhances the clarity of protein expression profiles and improves reproducibility between experiments.
Microdissected samples can be processed for a variety of analytical workflows, such as:
Mass spectrometry (MS): for identifying and quantifying the proteome.
Protein microarrays: to assess expression of hundreds or thousands of proteins simultaneously.
Western blotting or ELISA: for targeted protein validation.
By ensuring that only relevant cells are analyzed, researchers can better distinguish subtle molecular differences and obtain more accurate protein signatures.
Practical Considerations for LMD Success
For laser microdissection to yield high-quality results, several technical and procedural factors should be considered:
Section Thickness:
Preparing thin, consistent tissue sections (typically 5–10 microns) is essential for precise dissection and optimal molecular recovery. Thicker sections may impede laser focus or lead to incomplete separation.
Staining:
Minimal or specific histological stains can be used to identify target regions. Care must be taken to avoid stains or fixatives that interfere with downstream proteomic analysis. Some protocols use immunohistochemistry (IHC) or fluorescent markers to guide dissection.
Sample Quantity:
Although laser microdissection yields small quantities of tissue, modern proteomic techniques especially label free MS are capable of working with nanogram amounts of protein. Multiple microdissections can be pooled if needed.
Controlled Environment:
Performing LMD in a clean and temperature-stable environment reduces the risk of sample degradation. Specialized membranes, collection devices, and sterile tools are often used to maintain sample integrity throughout the process.
Summary
Laser microdissection represents a precise and effective approach for isolating specific cells or microregions from complex tissue sections. Whether working with FFPE or frozen tissues, LMD empowers researchers to analyze only the most relevant biological structures with minimal contamination and maximum molecular fidelity.
Its applications in proteomics are particularly valuable allowing for accurate protein profiling, reproducibility in multi-sample studies, and integration with high-throughput technologies like mass spectrometry and microarrays. When paired with optimized protocols and proper handling, LMD is a vital technique for anyone exploring the molecular landscape of biological tissues.